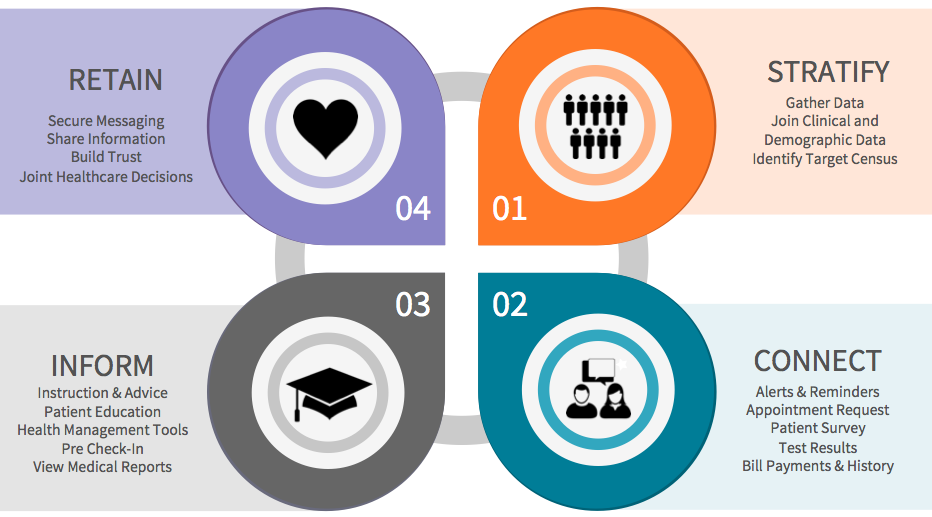
By March 31, 2017, all eligible laboratories must deliver private payor data and payor claims to CMS. The CMS will then be revising the Clinical Laboratory Fee Schedule (CLFS) for Medicare and Medicaid payment rates. According to Robert Michel, Editor-in-Chief of The Dark Report, this regulation "has the potential to trigger the most significant disruption to the clinical testing market place in four decades.”
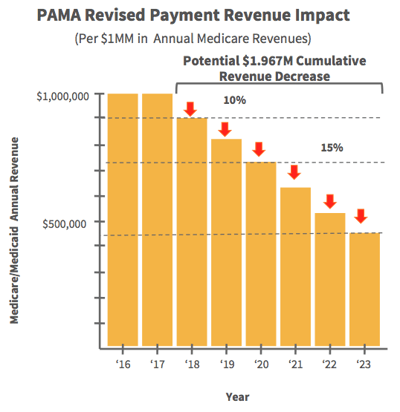
Many labs will not be able to handle the potential fines and fees associated with PAMA, including a $10,000/day penalty for late reporting data. PAMA is also expected to lower Medicare and Medicaid reimbursement rates by up to 15% over the next 7 years. This means it is imperative for labs to make decisions now that facilitate capturing new revenue, retaining existing revenue, and maximizing internal efficiency.
hc1 has put together a comprehensive executive guide that highlights how innovative laboratories can understand, prepare for, and survive PAMA regulations. We also included 3 proven strategies labs can use to prepare for PAMA, as well as stories about how leading labs have already started to prepare.
Download the entire Executive Guide here.